Application Spotlight: Pressure Switch Diaphragms for the Medical Industry
The medical device industry is driven by innovation, but highly regulated for reliability. And for healthcare, reliability isn’t a feature, it’s a requirement. While small, pressure switch diaphragms play a crucial role in life-saving and life-sustaining medical equipment. At Gulf Engineered Rubber and Plastics Solutions (Gulf), we specialize in designing and manufacturing custom pressure switch diaphragms that meet the stringent requirements of the healthcare industry.
The Role of Pressure Switch Diaphragms in Medical Devices
In medical devices that rely on accurate sensing, flow control, and pressure regulation, pressure switch diaphragms are essential. Common medical device applications that use pressure switch diaphragms include:
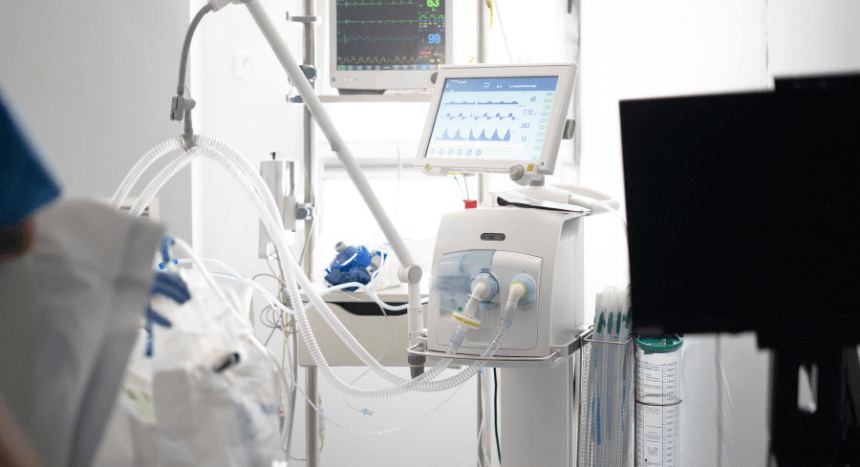
Oxygen concentrators and ventilators: In this medical device, the pressure switch diaphragm detects pressure changes that regulate airflow. It also ensures consistent oxygen delivery to the patient.
CPAP and BiPAP machines: The pressure switch diaphragm in a CPAP and BiPAP machine monitors and responds to pressure fluctuations. This way the machines maintain necessary airway pressure during sleep.
Blood pressure monitoring systems: A pressure diaphragm senses pressure changes. In a blood pressure cuff, it triggers measurements at the appropriate inflation and deflation points.
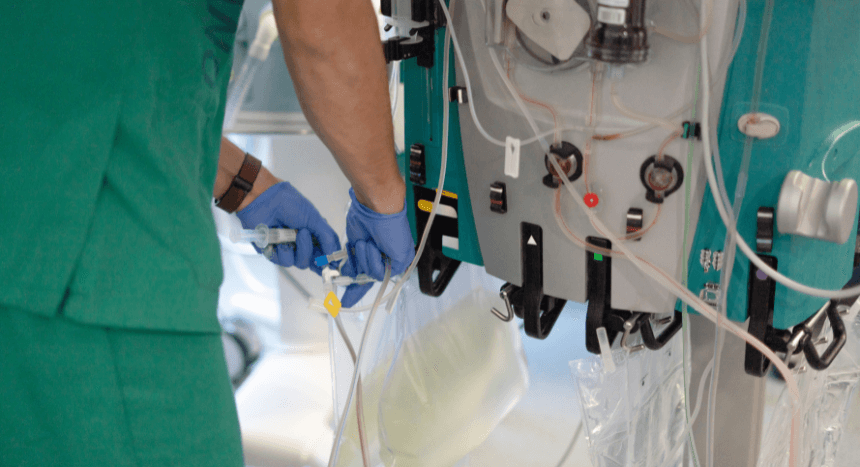
Infusion and drug-delivery pumps: Pressure switch diaphragms help control pressure in infusion and drug-delivery pumps. They ensure accurate and safe delivery of fluids or medication.
Sterile fluid handling systems: The pressure switch diaphragm maintains sterile barriers while at the same time monitors pressure to prevent leaks or contamination during fluid transfer.
As you can see, these devices depend on pressure switch diaphragms and their reliable response to minimal pressure changes, while also maintaining long-term stability under continuous use.
Unique Challenges for Pressure Switch Diaphragms in the Medical Industry
Medical environments present a unique set of challenges for pressure switch diaphragm design and manufacturing. This set of challenges is a prime example why selecting the right manufacturer is essential.
Biocompatibility
All materials used for medical devices must be non-toxic and compliant with relevant standards such as USP Class VI, ISO 10993, and FDA CFR Title 21, Part 177.2600 requirements. This ensures safe contact with human tissue or bodily fluids.
Precision and Sensitivity
Medical devices often require low actuation force and high sensitivity to detect micro-level changes in pressure which is critical in patient monitoring and therapeutic equipment.
Sterility and Contamination Control
Pressure switch diaphragms used in medical equipment are often manufactured and assembled in cleanroom environments to ensure sterility and prevent cross-contamination.
Fatigue Resistance
The continuous use in devices such as ventilators or infusion systems require the pressure switch diaphragm to handle long-term demand. Material fatigue resistance and lifecycle predictability are critical.
Material Selection for Medical Diaphragms
Choosing the right material is crucial to diaphragm performance in medical applications. Gulf works with a wide range of medical-grade elastomers, including:
Silicone (LSR & HCR): Excellent flexibility, thermal stability, and biocompatibility
EPDM: Resistant to heat, steam, and a wide variety of cleaning agents
FKM (Viton®): Superior chemical resistance for contact with aggressive media
TPE: For specific soft-touch and recyclable applications
PTFE-Laminated Elastomers: For chemical inertness and barrier protection
We also offer custom elastomer formulations developed in-house for unique performance requirements or regulatory standards.
Compliance & Cleanroom Manufacturing
At Gulf we understand that the medical industry demands not only performance but also documentation and validation. That’s why we offer:
- ISO-13485 certified manufacturing facilities
- Cleanroom production (ISO Class 7)
- Batch traceability and material certifications
- Support for FDA, CE, and global regulatory pathways
Our stringent QA protocols ensure your pressure switch diaphragms meet the tight tolerances and reliability standards demanded by medical applications.
Partnering with Gulf Rubber for Medical Applications
Whether you’re designing a drug delivery system or developing compact home healthcare devices, our team at Gulf offers a collaborative engineering approach to diaphragm development. Contact our team today to explore how we can help you bring safe, compliant, and innovative medical devices to life.